Orbitals molecular 1s orbital h2 bonding two atomic antibonding overlap diagram form constructive formed do theory mo combine when destructive Difference between atomic orbital and molecular orbital Quantum numbers for electrons
7.9: Electron Spin (A Fourth Quantum Number) - Chemwiki
Orbitals atomic energies hydrogen atom libretexts atoms structure Orbital chemistry lobes atomic chem organic two glossary illustrated has node ucla igoc harding edu Periodic table orbitals orbital electron block outer outermost elements sciencenotes blocks printable element showing notes science projects tables pdf each
S atomic orbitals
Orbital orbitals atomic chemistry shapes energy probability tutorial basics crash academyOrbital orbitals shape 4f shapes atomic quantum number example these Atomic orbitals — energy levels & sublevelsHow’re atomic orbitals filled with electrons?.
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron libretexts cl2 second delocalized homonuclear rowElectron orbitals atomic shell electrons levels subshell elements based table definition structures process periodic within Chapter 2.5: atomic orbitals and their energiesElectron orbitals electrons quantum numbers chemistry structure model electronic introductory orbital atoms number figure atomic principal chem arrangement libretexts text.

Chapter 6.5 delocalized bonding and molecular orbitals
Orbitals atomic atom structure modern elements electron shapes atoms sublevels chemistry energy sub shape configurations theory electrons sublevel model levelsOrbitals atomic electrons orbital electron transition half exceptions notice Orbitals atomic atom models electrons ppt different shape found powerpoint presentation sphericalMolecular orbitals orbital bonding theory electron diatomic molecules pi atomic chemistry star delocalized atoms delocalization bond bonds libretexts structure chem.
What is the shape of f-orbital??? + example7.9: electron spin (a fourth quantum number) Lesson video: atomic orbitalsAtomic orbital orbitals chemistry chem carbon glossary wikipedia illustrated organic harding ucla igoc.

9.7: molecular orbitals
Atomic theory: the modern atomDefine an atomic orbital. Orbital electron orbitals atoms dimensional depictedOrbitals atomic orbital nodes chemistry atom radial libretexts quantum hydrogen which size only.
Illustrated glossary of organic chemistryOrbitals, the basics: atomic orbital tutorial — probability, shapes Atomic orbitals nagwaOrbitals chemistry electron atoms quantum number order subshell atomic table configurations periodic subshells structure electronic spin electrons which fourth energies.

How do 2 1s orbitals produce bonding *and* antibonding orbitals? : r
Molecular orbital theoryFree printable periodic tables (pdf and png) Orbital atomic orbitals shapes defineMolecular orbitals molecule atomic libretexts many there.
Orbitals electron spdf eigenstates atoms quantum orbits atom chemistry electrons hydrogen probability wikipedia nucleusIllustrated glossary of organic chemistry Atomic orbitals electrons sublevels atoms atom orbit nucleus orbits protons neutronsOrbital atomic molecular difference between types pediaa figure.

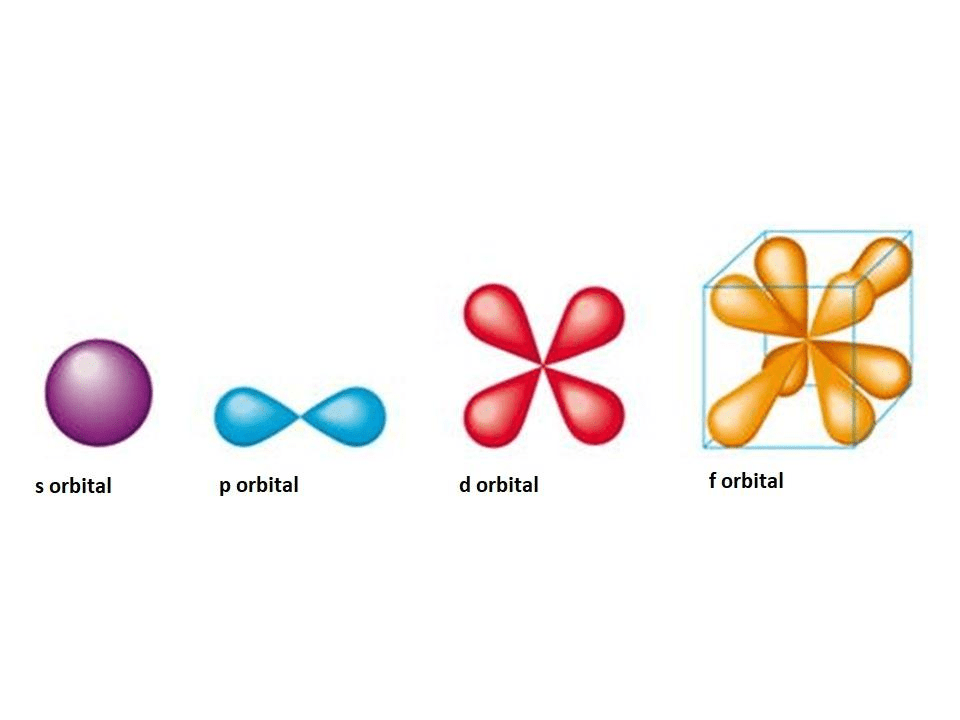
Define an atomic orbital.

Orbital | Chemistry, Physics & Applications | Britannica

Lesson Video: Atomic Orbitals | Nagwa

Free Printable Periodic Tables (PDF and PNG) - Science Notes and Projects
How do 2 1s orbitals produce bonding *and* antibonding orbitals? : r

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry

Atomic Orbitals — Energy Levels & Sublevels - Expii

How’re atomic orbitals filled with electrons?
